A. Fick’s Law
Molecular diffusion is defined as the transfer or the travelling in a random path of molecules of A through molecules of B due to concentration gradient.

|
8th Handbook, p:5-45, Eq. 5-189 |
C – the total concentration of A and B DAB – diffusivity of A through B xA – the concentration of A at some point z – direction of net transfer of A |
B. Diffusivity
1. Diffusion coefficients of Binary mixture at 1 atm are listed in 8th Handbook, p:2-454, T:2-324 .

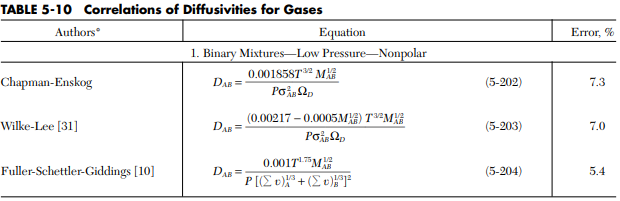
2. Correlation of Diffusivity for Gases in 8th Handbook, p:5-51, T:5-10 – correlates the diffusivity to parameters such as temperature and pressure.
C. General Cases for Gases
1. Equimolar Counter Diffusion
– a two-way diffusion where the total net flux is zero. This implies that the net flux of A is balanced by a counter net flux of B such that the total concentration will not change.
|
8th Handbook, p:5-49, Eq:5-197 |
C – the total concentration of A and B DAB – diffusivity of A through B xR – the concentration of A at Right Side xL – the concentration of A at Right Side |
For gases, constant concentration is maintained if P and T remain constant.
By employing ideal gas equation for appreciably lower P, the equation will take the form of:
|
|
DAB – diffusivity of A through B yR – the concentration of A at Right Side yL – the concentration of A at Right Side P – absolute pressure of the gas |