Distillation is a unit operation employed to purify or to separate components in a mixture on the basis of their volatility. More volatile component vaporizes faster than less volatile component; hence, separation is feasible.
I. Principles of Distillation
A. Vapor Pressure
Vapor Pressure is the pressure exerted by the vapor phase in equilibrium with the liquid phase. Several correlations can be utilized to determine for the vapor pressure of a particular compound. The most familiar is the Antoine Equation, 8th Perry’s Handbook, T 13 -14 ,p. 13-14, because of its simple form. See figure below.
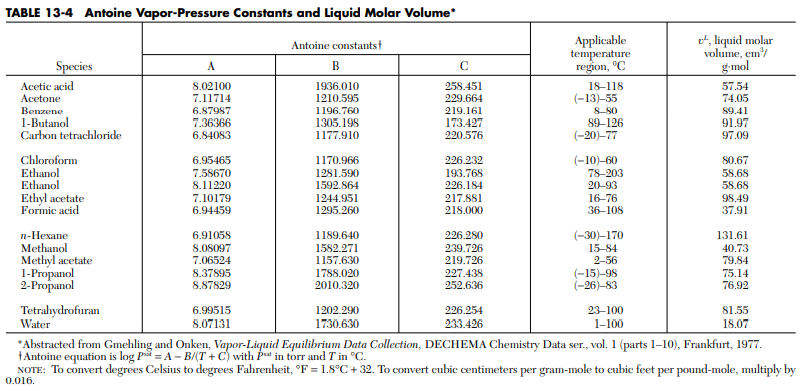
A more extensive way of calculating the Vapor pressure of pure substances is presented in 8th Perry’s Handbook, T 2 – 8 p. 2-55 .
| p in Pascal (Pa) T in Kelvin (K) |
For ideal solutions, the vapor pressure (partial pressure) exerted of every volatile component deviates from the pure vapor pressure by a factor only: Raoult’s Law (Vapor Pressure Lowering) .
B. Boiling
When a pure substance is heated, it will boil at a particular temperature for a given pressure. For example, water boils at 100 oC at atmospheric pressure. However, this is not the case with solutions. At different concentrations, the mixture will start to boil at different temperatures.
Bubble Point Temperature is the temperature of the mixture when a first bubble of vapor occurs as the components of a mixture exerted vapor pressures whose sum equals the operating pressure. This boiling temperature is intermediate between the boiling temperatures of the pure components.

Figure above depicts the Vapor-liquid Equilibrium relation of Pentane-Hexane Mixture at 1 atm. Tie lines of a binary mixture connects the liquid and vapor concentrations in equilibrium and are drawn horizontally.
Example 1:
Determine the bubble point temperature at atmospheric pressure of cold pentane-hexane mixture if the concentration of pentane is 0.45.
Solution:

Vapor Pressure of Inorganic and Organic Liquids: 8th Perry’s Handbook, T 2-8, p. 2-55
| Pentane | Hexane | ||
|---|---|---|---|
| C1 | 78.741 | C1 | 104.65 |
| C2 | -5420.3 | C2 | -6995.5 |
| C3 | -8.8253 | C3 | -12.702 |
| C4 | 9.6171E-06 | C4 | 1.2381E-05 |
| C5 | 2 | C5 | 2 |

Evaluate T by iteration, or use a sophisticated calculator.
T = 323.09K = 49.94 oC ![]() 50 oC
50 oC
Referring to the pentane-hexane plot in table above for xh = 0.45, the Bubble Point is approximately 50 oC.
C. Relative Volatility, 
Distribution coefficient factor, K , is the concentration ratio in the vapor to the liquid in equilibrium – 8th Perry’s Handbook, Eq. 13 – 1, p. 13 – 2
![]()