Evaporation is a unit operation of concentrating a solution of solvent and non-volatile solute by evaporating the solvent, usually water. The product can either be derived from the remaining concentrated liquid or from the evaporated solution.
A. Duhring Rule
For strong solutions, the properties deviate greatly from that of the pure substances. Specifically, the boiling point of the solution deviates from the boiling point of the pure solvent. We call this particular deviation as Boiling Point Elevation. Duhring Rule shows that there is a linear relationship between the boiling point of the pure solvent and the solution for a given pressure. Below are the sample plots of Duhring Lines.
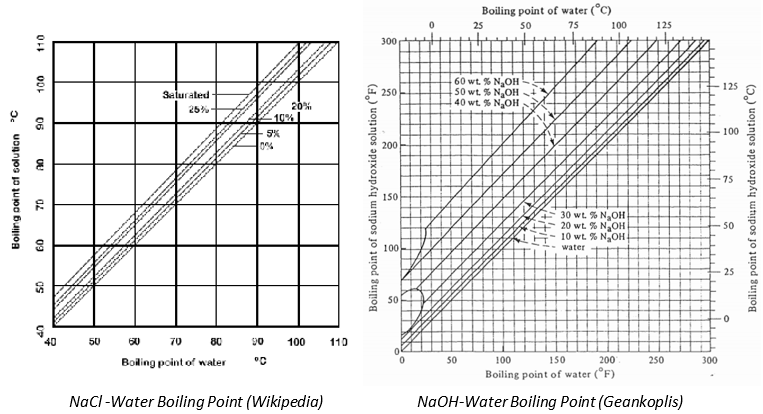
Perry’s handbook has a construction of nomograms to determine the boiling point of various aqueous solutions.
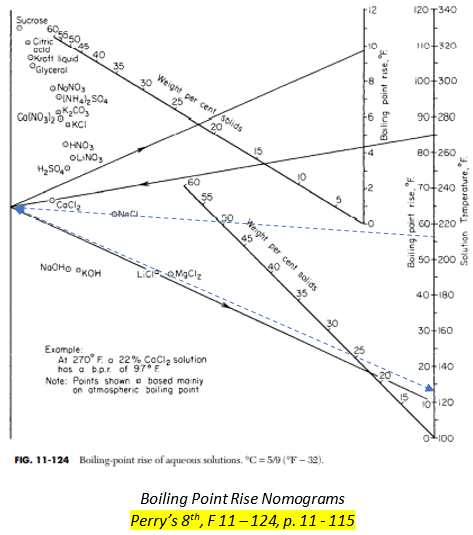
Steps to determine the BPR(Boiling Point Rise) using Perry’s Handbook Nomogram
In the figure above, a sample was provided for 25% NaCL solution subjected to pressure equivalent to boiling temperature of water at T = 200 oF.
1. For a given saturated temperature of a pure solvent, approximate the boiling point temperature on the right side.
Assume Tb as 212oF.
2. Connect a line, passing through the required aqueous solution, to the left side.
3. Draw another line starting at the end of the first line passing through the weight per cent solids of the solution and ending on the Boiling point rise, BPR.
BPR is approximately 13.
4. Add the BPR to the given saturated temperature and check if it is equal to the approximated Boiling Point Solution.
Tb = 200 + 13 = 213
5. If not equal, repeat the steps for different approximate boiling point.
The approximated boiling point is nearly equal to the derived boiling point. 212 ![]() 213. Accept the value.
213. Accept the value.
Similarly, the enthalpy of the solution will vary. For common solutions, enthalpy – concentration diagrams are also provided.
